- Shanghai Zhongshen International Trade Co., Ltd. - Two decades of trade agency expertise.
- Service Hotline: 139 1787 2118
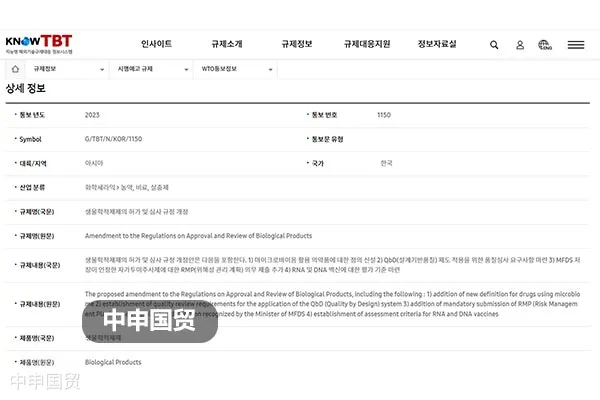
On June 26, 2023, the Ministry of Food and Drug Safety (MFDS) of South Korea submitted Notification G/TBT/N/KOR/1150 to the WTO, proposing revisions to the Regulations on Licensing and Review of Biological Products, etc. The goal of the revisions is to further strengthen the safety of domestic biological products. As of August 25, 2023, these revisions are in the public consultation phase.
China is the world's second-largest biologics market, and the export scale of related products has been increasing year by year. In 2022 alone, China's pharmaceutical exports to South Korea reached $1.257 billion. Currently, South Korea is the top export destination for China's "Western medicine preparations," making this revision highly significant for Chinese export enterprises. It is recommended that export enterprises pay close attention to the content changes in this revision, promptly adjust product strategies, and avoid unnecessary trade losses in future exports.
Summary of revisions:
(1) Definition of new terms: Definitions for new terms such as live bacterial preparations;
(2) Quality system audit requirements: Established quality audit requirements for Quality by Design (QbD) systems (Articles 4 and 26 of the proposal);
(3) Expansion of the "Scientific Citation Index" Scope: The range of materials recognized as pharmacological effects and clinical trial efficacy data under the "Scientific Citation Index" has been expanded (Article 7 of the Plan);
(4) Improved stability test data submission requirements: Changed the submission requirements for stability test data when modifying manufacturing methods;
(5) Risk Management Plan (RMP) submission procedures: Added mandatory RMP submission procedures for self-injection products approved by MFDS;
(6) RNA and DNA vaccine evaluation standards: Established evaluation standards for RNA and DNA vaccines.
Related Recommendations
? 2025. All Rights Reserved. Shanghai ICP No. 2023007705-2  PSB Record: Shanghai No.31011502009912
PSB Record: Shanghai No.31011502009912